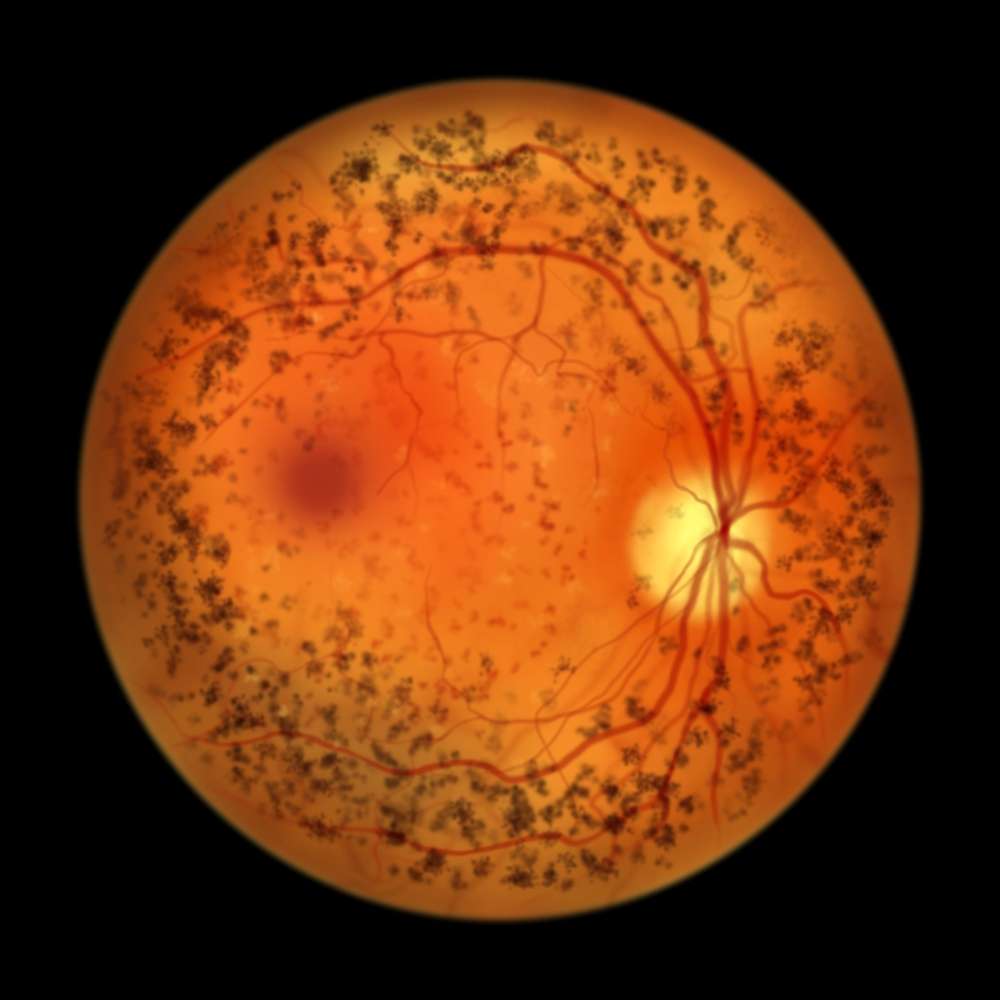
Apellis reports rare retinal vasculitis in patients with pegcetacoplan injection
Apellis Pharmaceuticals has presented an update on rare incidences of retinal vasculitis observed in patients treated for geographic atrophy with their medication pegcetacoplan injection. Apellis undertook a thorough evaluation of the manufacturing process and medication product and discovered no flaws that might have led to the occurrence of retinal vasculitis. Between clinical studies and commercial supply, there were no modifications in the medicine formulation, and no quality concerns or impurities were detected. There was no indication of drug-related immunogenicity in the clinical trial data, and no specific manufacturing lot was involved. Apellis has confirmed seven incidences of retinal vasculitis in individuals treated with pegcetacoplan injection since its inception. There were two cases in April, two in May, and three in June.
To know more: About the original article click here.
