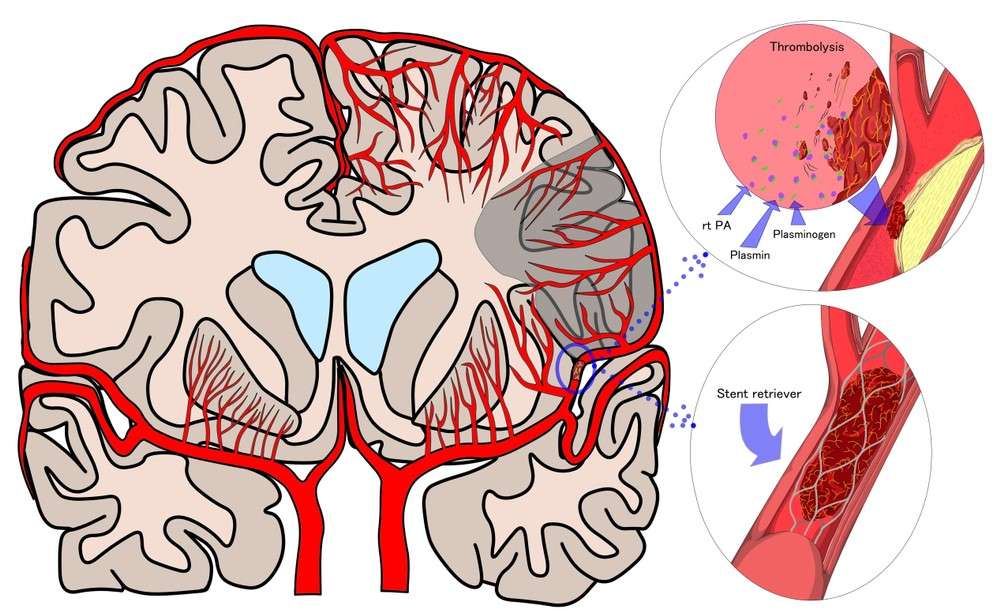
BAST study evaluates neuroprotective medication for acute ischemic stroke
The BAST clinical study looked at the efficacy and safety of the neuroprotective medication DL-3-n-butylphthalide (NBP) in treating acute ischemic stroke. The study discovered that when paired with reperfusion treatment, NBP resulted in a larger proportion of good functional outcomes after 90 days than placebo. The NBP and placebo groups had no significant differences in adverse events. The study’s drawback was the small number of patients who got endovascular therapy, which limited the generalizability of NBP use in these people. Despite earlier failures with other neuroprotective efforts, the BAST study appears to be promising in terms of improving functional outcomes for stroke patients. The experiment took place in China from July 2018 to May 2022, and 1216 people were diagnosed with acute.
To know more: About the original article click here.
