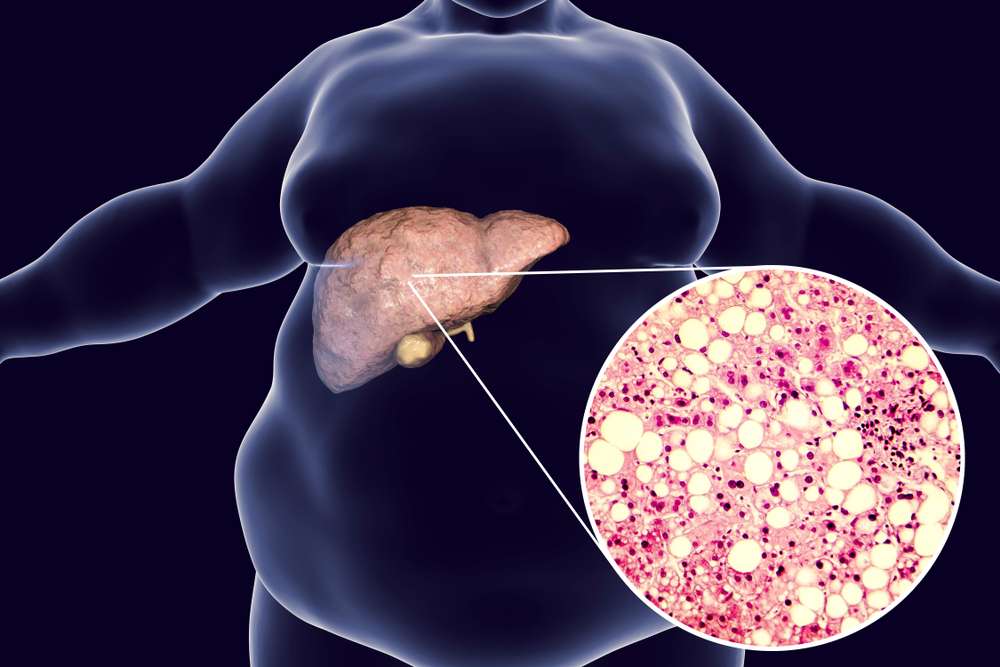
Tern Pharma’s TERN-501 in phase 2a study of nonalcoholic steatohepatitis
TERN-501, an oral thyroid hormone receptor beta agonist developed by Tern Pharmaceuticals, was reported in a phase 2a study to reduce liver fat content in individuals with nonalcoholic steatohepatitis. The medicine was administered either alone or in conjunction with TERN-101, an FXR agonist. TERN-501 demonstrated dose-dependent and statistically significant decreases in liver fat as evaluated by MRI, with the 6 mg dosage lowering liver fat by 45% against 4% for placebo. A higher proportion of TERN-501 patients had at least a 30% decrease in liver fat, which was associated with improvements in NASH. TERN-501 also decreased LDL, HDL, and triglyceride levels. The combination of TERN-501 and TERN-101 increased liver fat removal somewhat more than TERN-501 alone. Importantly, TERN-501 had a favorable safety profile, with very minor adverse effects observed.
To know more: About the original article click here.
