Automatic Insulin Delivery Systems Improves Glycemic Control
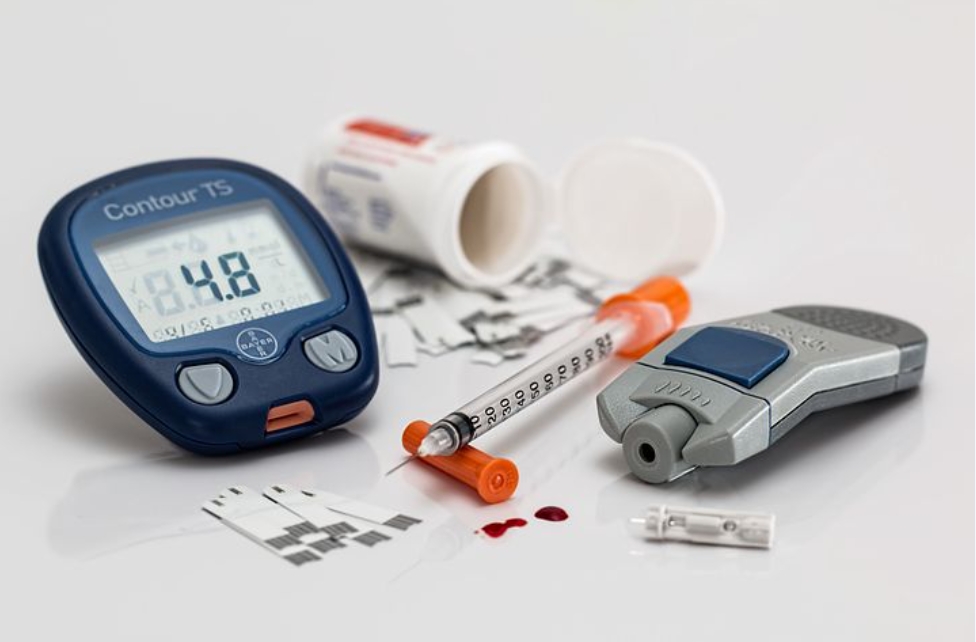
Automatic Insulin Delivery systems are automated (or semi-automated) systems designed to assist people with diabetes, primarily type 1, by automatically adjusting insulin delivery to help them control their blood glucose levels. The development of automated insulin delivery has many names artificial pancreas, hybrid closed loop, bionic pancreas, but all share the same goal: using continuous glucose monitors (CGM) and smart algorithms that automatically adjust insulin delivery via pump.
There are three automatic insulin delivery systems on the U.S. market, two of which are currently approved by the U.S. Food and Drug Administration. These systems have already made a significant impact for the people who use them in improving diabetes outcomes, including glycemic control and hypoglycemia prevention.
We now have better insulins with more consistent absorption (including peak less basal insulins), insulin pumps, continuous glucose monitoring (CGM) systems, and, more recently, even algorithms that tie these devices together to achieve automated insulin delivery (AID).
With a hybrid closed-loop system, individuals spend less time manually controlling their diabetes management. A sensor tracks their blood glucose levels (or the sensor glucose levels), and then an insulin pump responds and doses an appropriate amount of insulin as needed. This takes a lot of the stress and burden off of individuals, especially overnight.
The FDA evaluated data from a clinical trial of the MiniMed 670G hybrid closed looped system that included 123 participants with type 1 diabetes. The clinical trial included an initial two-week period where the system’s hybrid closed loop was not used followed by a three-month study during which trial participants used the system’s hybrid closed loop feature as frequently as possible. This clinical trial showed that the device is safe for use in people 14 years of age and older with type 1 diabetes. No serious adverse events, diabetic ketoacidosis (DKA), or severe hypoglycemia (low glucose levels) were reported during the study.
Risks associated with use of the system may include hypoglycemia, hyperglycemia, as well as skin irritation or redness around the device’s infusion patch. This version of this device is unsafe for use in children 6 years of age or younger and in patients who require less than eight units of insulin per day.
FLAIR enrolled 113 patients with T1D at seven international diabetes centers. The participants, adolescents and young adults, ages 14 to 29, used each AID system for three months in a randomized crossover trial.